Research
Modern theoretical knowledge of medicine should be obtained where there is modern scientific research.
Leading medical schools around the world have traditionally been research centers. We want this tradition to take root in Ukraine as well.
Research activities are a key focus area for Dobrobut Academy Medical School. Research is an integral part of medical training. Dobrobut Academy Medical School is the first and only medical school in Ukraine that combines research, teaching and practice to give its medical interns (now) and medical students (planned in 2026) access to the best medical education that prepares them for actual clinical practice. Our teaching staff engage in world-level scientific research to provide our medical interns and students with the most modern medical education.
In this context, we are actively developing the Research Center of the Dobrobut Academy Medical School - a key element in the development of full-fledged university research.
That is why we are building a research centre on the basis of the “Dobrobut Academy”
What will be happening there?
- Support for scientific research of the Dobrobut team
- A platform for research cooperation and implementation of research within international and national projects
- Science park - opportunities for preclinical and clinical trials based on the laboratory of the Wellbeing Academy, development of innovative projects in medicine
The structure of the centre includes:
- Laboratories for bioengineering, medical engineering and pain mechanisms research
- Behavioural testing and molecular biology modules
- Auxiliary facilities, including seminar rooms and a recreation area
Ongoing projects:
Although a relatively young institution, DAMS has demonstrated strong research leadership. Key projects led or co-led by the DAMS' PIs include:
- 2022–2025: Effective Treatment of Peripheral Nerve Injury by Biomimetic 3D Scaffolds. Funded by the National Research Foundation of Ukraine (Grant No. 2021.01/0328). Total budget - UAH 9,998,400
- 2019–2025: The Role of the Complement System in Spinal Mechanisms of Chronic Pain (NIH R01 NS113189). Co-PI: Prof. Nana Voitenko (PI - Yuriy Usachev, Iowa Uneversity, USA). Ukrainian budget: $709,513.
- 2019–2022: Pan-European Twinning to Re-establish World-Level Neuroscience Centre in Kyiv (NEUROTWIN). Horizon 2020 CSA project coordinated by Prof. Nana Voitenko. Total budget: €779,562.

The main areas of work of our laboratories:
SPINAL AND PERIPHERAL IMPLANTS PRODUCED BY HIGH SPATIAL RESOLUTION 3D PRINTING
In the last few years, there has been a growing interest in studying the mechanisms of regeneration of nervous tissue damage, in particular peripheral nerves and the spinal cord. Injuries and degenerative damage to these nervous tissues are quite common as a result of road accidents, industrial injuries, combat, and neurodegenerative diseases. Spinal cord injuries lead to a loss of functional activity of the body below the site of injury, which affects a person's ability to self-care and significantly reduces their ability to work. The consequences of spinal cord injuries cause significant social and economic losses every year in all countries of the world. The development of new methods of treating central nervous system pathology requires mandatory preliminary testing of their effectiveness in both in vitro and in vivo experiments. That is why the search for and creation of an optimal comprehensive model of spinal cord injury in animals of different sexes, which would best match the holistic picture of the injury typical of real conditions in humans, is an urgent task for our laboratory.
We propose an innovative biotechnological approach for 3D printing implants of arbitrary shape with an internal structure of the required level of detail with a spatial resolution of up to several micrometres. This approach is based on the photopolymerisation of synthetic polymers or modified proteins. Two-photon microscopy (2P-microscopy) or digital light processing (DLP, Digital Light Processing) are or will be used as engineering approaches for 3D printing implants. The implants are designed and manufactured to be used as a supportive framework for major structural components of the nervous and vascular systems.
This innovative biotechnological approach, which involves the formation of spatially oriented fragmented matrices and their implantation into the locus of peripheral nerve or spinal cord injury together with neural crest stem cells, mesenchymal stem cells or neural stem cells, is expected to provide functionally complete implants. They will help to significantly improve both afferent and efferent communication through the site of damage and regenerate local networks in the hind and anterior horns. The effectiveness of this approach will be tested by studying the functional activity of paretic limbs, especially in the late period of the recovery process.
This approach (in combination with optogenetic, viral, genetic and immunocytochemical studies) will provide a unique opportunity for precise electrophysiological monitoring of newly formed nerve tissue. The studies meet the criteria for preclinical research, and their positive results can be extended to clinical trials, especially in aspects related to rehabilitation after peripheral nerve injury. This innovative method of treating spinal cord and peripheral nerve injuries using tissue engineering is expected to be introduced into the experimental protocols of public and private clinics in Ukraine that perform surgical treatment of spinal cord and peripheral nerve injuries.
- The project received funding from the National Research Foundation - grant 2021.01/0328 ‘Effective treatment of peripheral nerve injuries with biomimetic 3D implants’
- This project is being developed in cooperation with scientists from:
- The Katholieke Universiteit Leuven (or KU Leuven, Leuven, Belgium ) https://www.kuleuven.be
- Romodanov Neurosurgery Institute NAMSU (Kyiv, Ukraine) https://neuro.kiev.ua
- Kyiv Academic University (Kyiv, Ukraine) https://kau.org.ua/deps/biph
- Bogomoletz Institute of Physiology (Kyiv, Ukraine) http://biph.kiev.ua
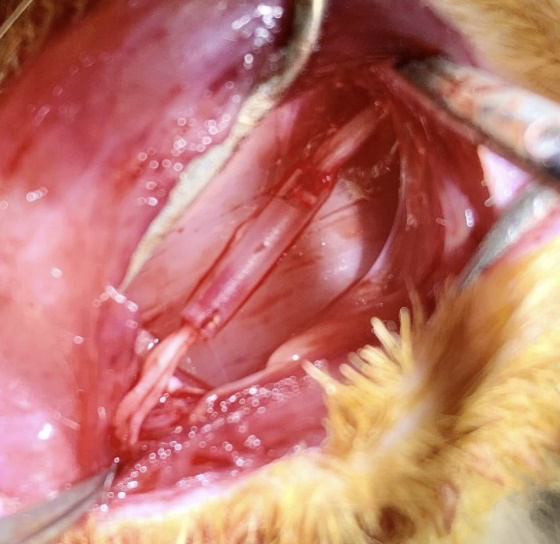
Scaphold implantation into the sites of peripheral nerve damage
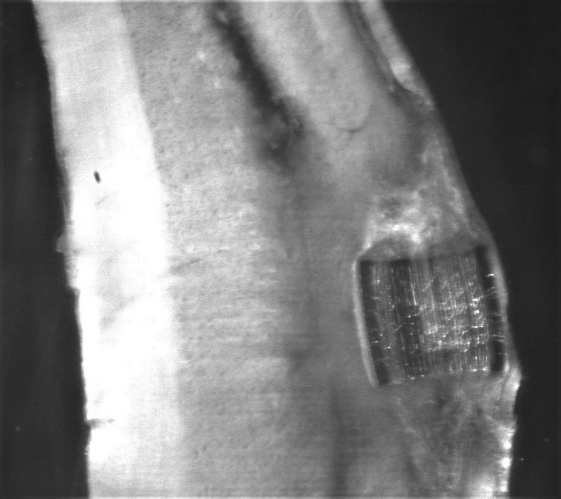
Scaffold for implantation in spinal cord injuries
DEVELOPMENT OF THE LATEST ELECTROPHYSIOLOGICAL AND OPTICAL METHODS IN BIOMEDICAL RESEARCH. MICROSCOPY OF INTACT BIOLOGICAL PREPARATIONS
Over the past decade, the imaging of unstained, live cells of intact tissue of various types has been an urgent problem. This is especially important for ex vivo and in vivo electrophysiological studies. More and more often, side-view infrared LED illumination is used in biomedical research. However, there is no commercial and high-quality equipment of this type. The use of short-wave and long-wave LEDs as side illumination sources is a good combination of approaches. It is possible to combine high-resolution images of surface neuronal structures such as axons and dendrites (blue LED) with images of soma cells deep within the preparation (infrared LED, 850-1000 nm).
The aim of our work is to develop a new type of condenserless compact microscope with variable intensity LED control modules to obtain high-resolution images of intact biological structures at a depth of 100-150 µm inside the preparation.
This project is being developed in cooperation with scientists from:
- Institute for Molecular and Cell Biology (Porto, Portugal) https://www.i3s.up.pt/research-group?x=40
- Kyiv Academic University (Kyiv, Ukraine) https://kau.org.ua/deps/biph
- Bogomoletz Institute of Physiology (Kyiv, Ukraine) https://biph.kiev.ua
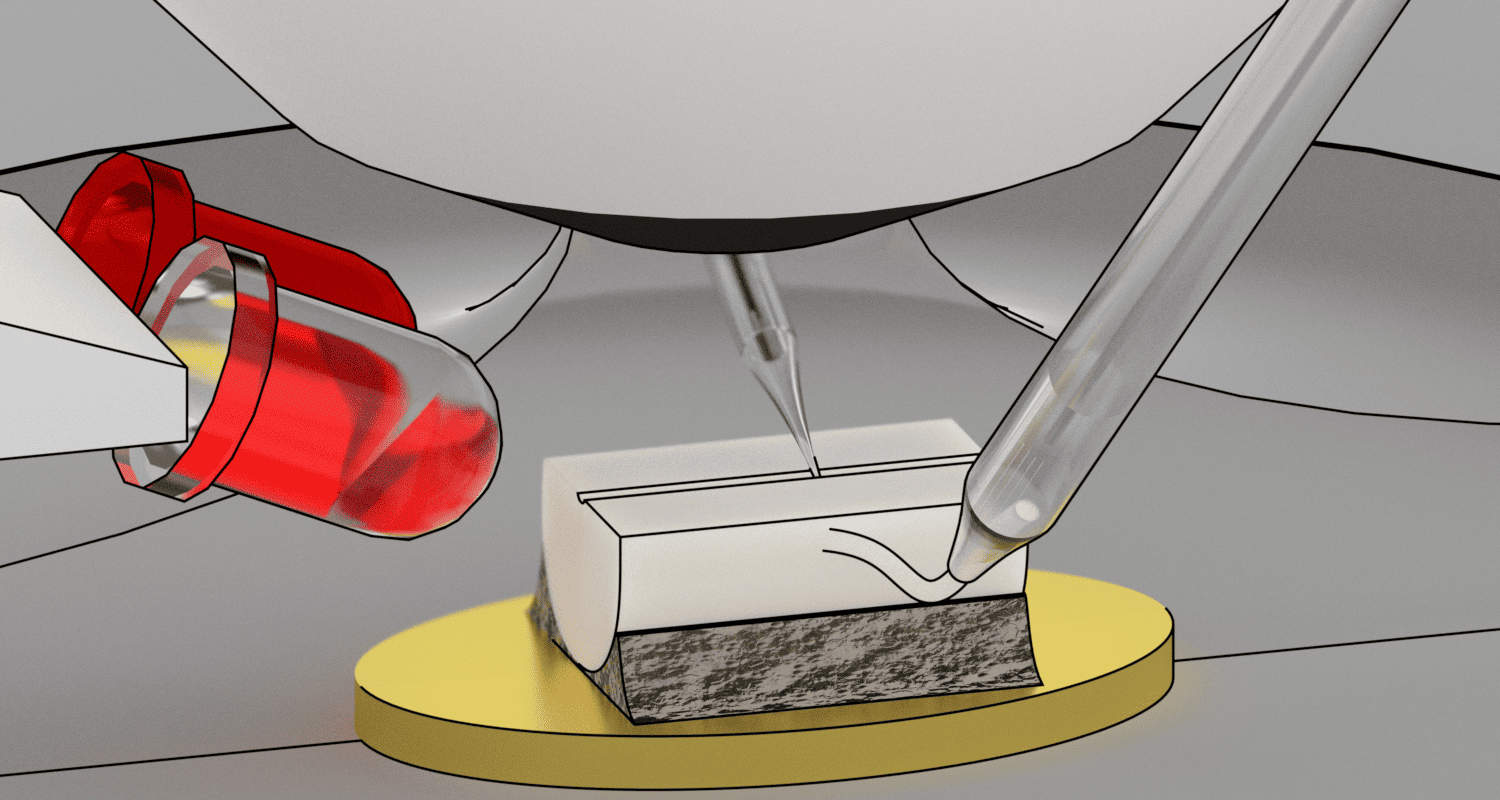
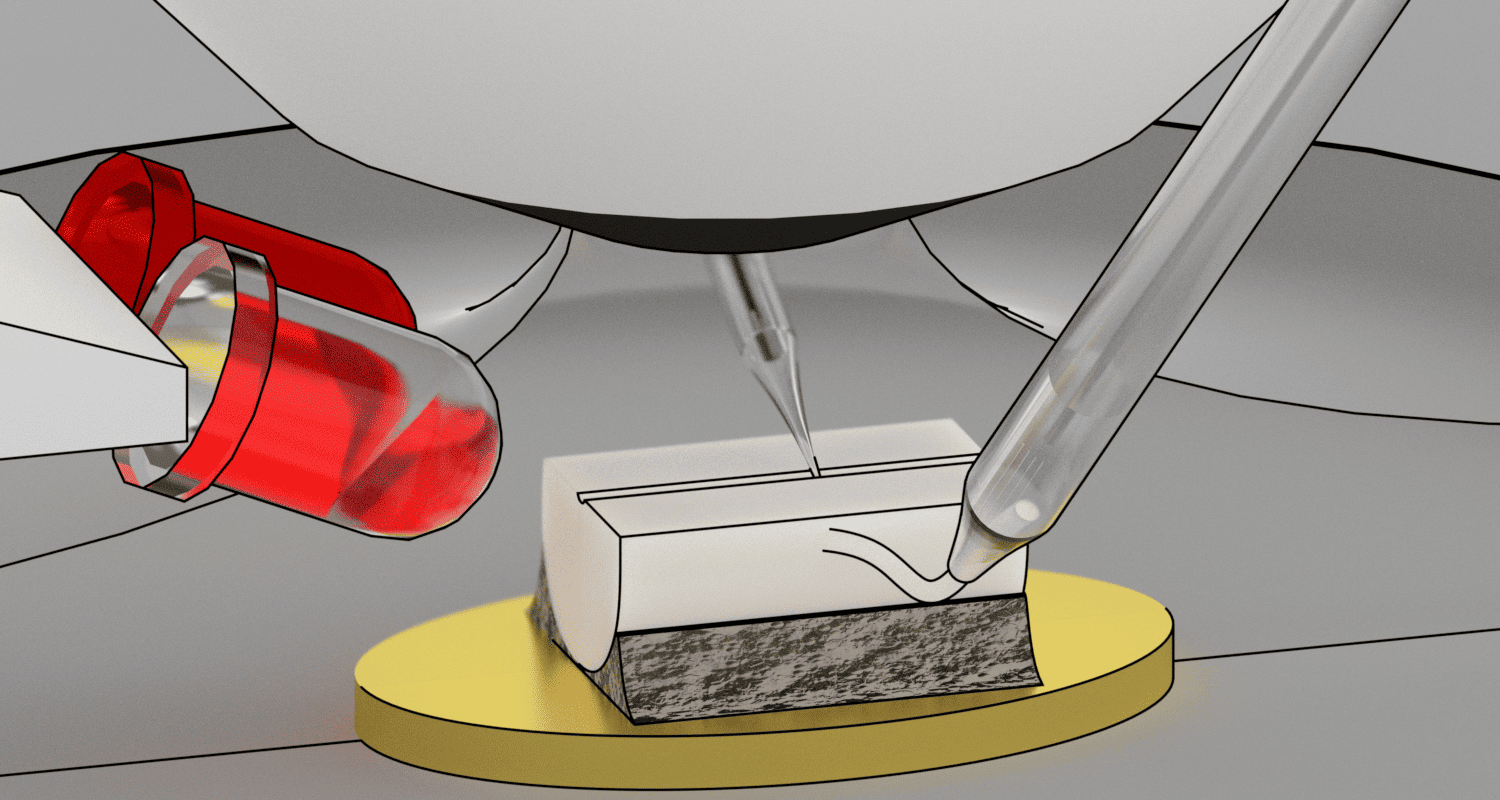
Diagram of the adaptation of a standard microscope for the study of lamina X cells of the rat spinal cord using side infrared illumination.
DEVELOPMENT OF JOINT ELECTROPHYSIOLOGICAL AND OPTICAL APPROACHES FOR THE STUDY OF INTACT BIOLOGICAL PRODUCTS
The new type of capacitorless compact microscopes requires significant changes to adapt the electrophysiological, fluorescence optical, optogenetic and ancillary equipment used for ex vivo and in vivo studies.
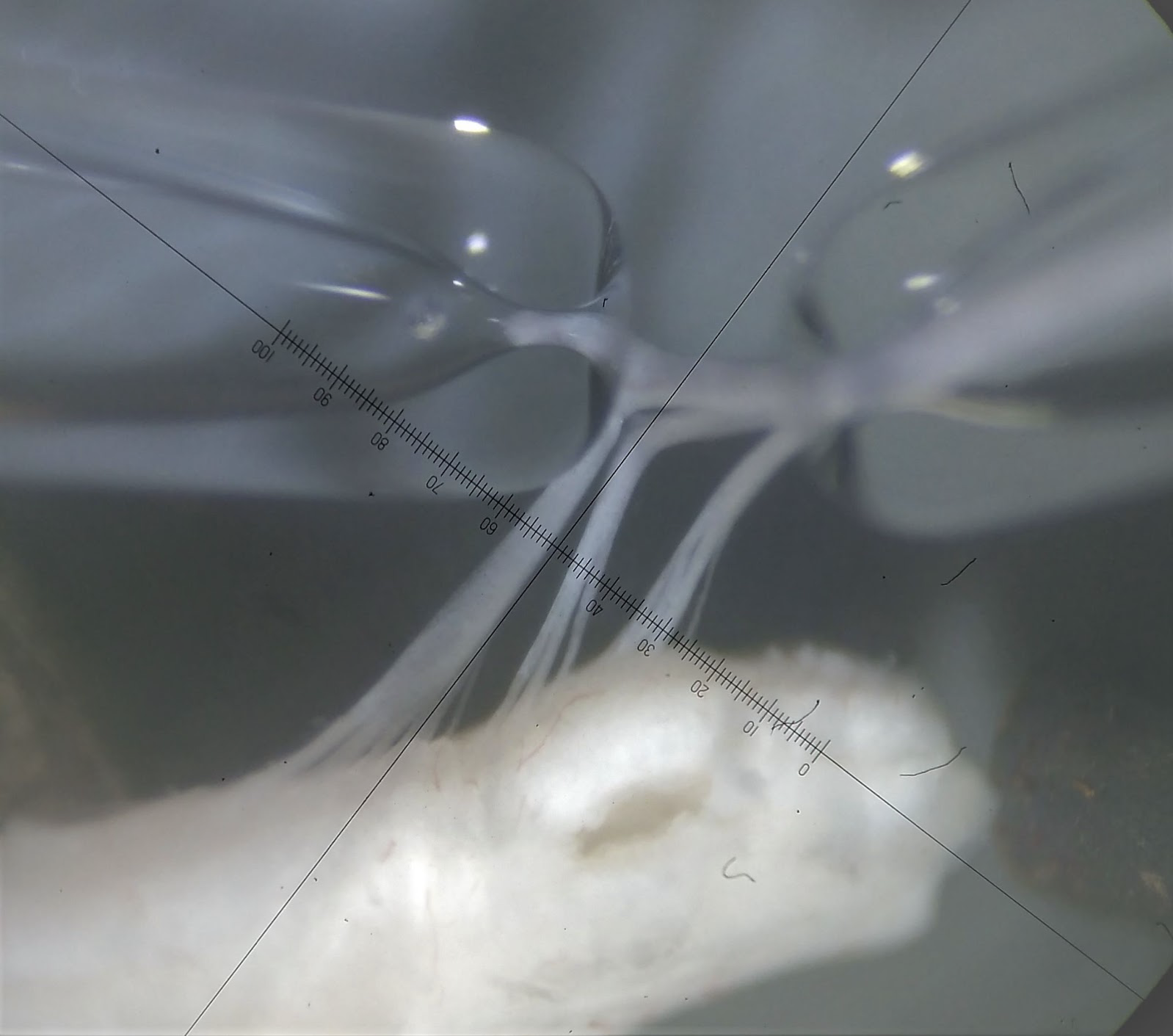
Images of the spinal cord and dorsal root ganglia being stimulated and recorded using glass pipettes. Images were acquired using side illumination on a standard Olympus BX50WI research microscope.
 An experimental setup that combines short-wave and long-wave LEDs as sources of side illumination, as well as electrophysiological and optogenetic equipment.
An experimental setup that combines short-wave and long-wave LEDs as sources of side illumination, as well as electrophysiological and optogenetic equipment.
DEVELOPMENT OF NEW THERAPEUTIC STRATEGIES FOR THE TREATMENT OF CHRONIC PAIN
Persistent or chronic pain caused by inflammation, infection, tissue damage or nerve damage is one of the major health problems worldwide. Chronic pain affects about 10% of the population of developed countries in Europe and North America and results in a loss of more than $100 billion a year.
In this project, we propose a new hypothesis that in chronic inflammatory pain, activated PKCα leads to an increase in the number of Ca2+-permeable AMPA receptors in the neurons of the dorsal horn of the spinal cord, which is critical for the induction and maintenance of chronic inflammatory pain. Based on the above molecular mechanism, we propose to develop a new therapeutic approach to the treatment of chronic pain.
This approach is based on local specific blocking of PKCα activity in the neurons of the lumbar spinal cord. This allows to correct changes in AMPA receptor transport caused by peripheral inflammation of the hind limbs and thereby block or reduce chronic inflammatory pain. Most likely, due to local and specific genetic blocking, this therapeutic approach will not be accompanied by side effects.
This project is being developed in cooperation with scientists from:
- Institute for Molecular and Cell Biology (Porto, Portugal) https://www.i3s.up.pt/research-group?x=40
- University of Iowa (Iowa City, USA) https://medicine.uiowa.edu/pharmacology/profile/yuriy-usachev
- Kyiv Academic University (Kyiv, Ukraine) https://kau.org.ua/deps/biph
- Bogomoletz Institute of Physiology (Kyiv, Ukraine) http://biph.kiev.ua
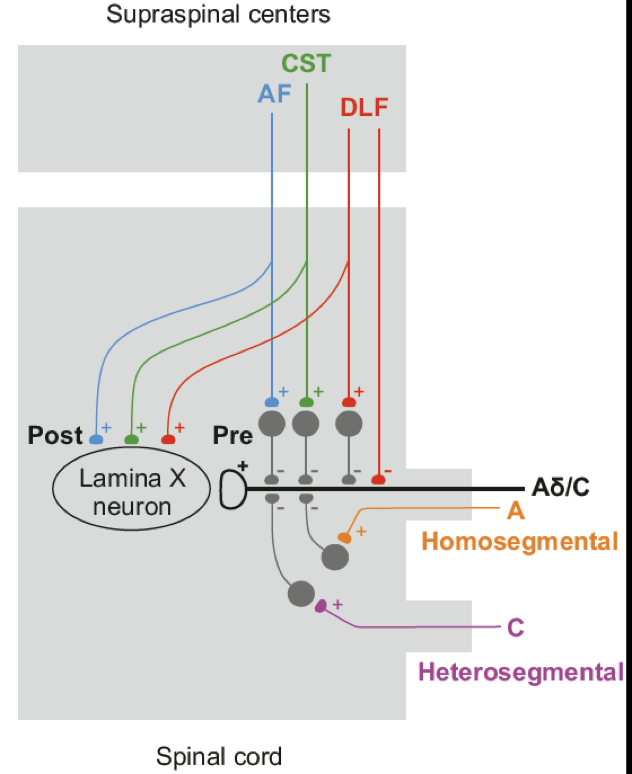
We propose a scheme of downstream regulation of nociceptive inputs to lamina X.
Behavioural test module
This laboratory uses methods to study animal behavioural responses. This allows us to investigate the relationship between changes in molecular mechanisms at the cellular level and certain disorders of animal sensory sensitivity at the organismal level.
The main areas of work of the laboratory:
- Testing of animals before and after implantation in case of spinal cord and peripheral nerve injury
- Testing animals with chronic pain syndrome before and after treatment using pharmacological or genetic approaches.
Our laboratory is equipped with such equipment:
- Hargreaves apparatus
- Von-Frey filaments
- Rotarod open-field
- Hot-plate test
- Randall–Selitto test
Molecular biology module
In order to create the most therapeutically suitable implants (in terms of materials, architecture, tropism, biocompatibility, degradation, the possibility of their molecular and cellular functionalisation, etc.), various types of research are required. These include immunocytochemical (fluorescence microscope), molecular genetic (RT-PCR, Western blot), in vitro (studying the interaction of matrix materials with various cell types, selection and functionalisation of cells prior to their placement in the implant, etc.) and in vivo (tracing upstream and downstream pathways within the animals themselves using viruses and dextrans).
Our Scientific Team:
- Nana Voitenko, Ph.D., Doctor of Science in Physiology, Professor of Biophysics. Principal Research Scientist
- Volodymyr Medvedev, MD, Ph.D., Doctor of Science in Neurosurgery. Senior Researcher
- Taras Petriv, MD, Ph.D. Researcher
- Oleksandr Bomikhov, PhD Student, Research associate
- Valeriia Ustimenko, PhD Student, Research associate
- Yana Naumenko, PhD Student, Research associate
Scientific publications
- Kopach O, Pivneva T, Fedirko N, Voitenko N. (2024) Mitochondrial malfunction mediates impaired cholinergic Ca2+ signalling and submandibular salivary gland dysfunction in diabetes. Neuropharmacology. 2024 Feb 1;243:109789. doi: 10.1016/j.neuropharm.2023.109789. Epub 2023 Nov 14. PMID: 37972885.
- Ivasiuk A, Matvieienko M, Kononenko NI, Duzhyy DE, Korogod SM, Voitenko N, Belan P. (2023) Diabetes-Induced Amplification of Nociceptive DRG Neuron Output by Upregulation of Somatic T-Type Ca2+ Channels. Biomolecules. 2023 Aug 28;13(9):1320. doi: 10.3390/biom13091320. PMID: 37759720.
- Krotov V, Agashkov K, Romanenko S, Koroid K, Krasniakova M, Belan P, Voitenko N. (2023) Neuropathic pain changes the output of rat lamina I spino-parabrachial neurons. BBA Adv. 2023 Feb 14;3:100081. PMID: 37082260.
- Kopach O, Dobropolska Y, Belan P, Voitenko N. (2023) Ca2+-Permeable AMPA Receptors Contribute to Changed Dorsal Horn Neuronal Firing and Inflammatory Pain. Int J Mol Sci. 2023 Jan 25;24(3):2341. PMID: 36768663.
- Krotov V, Medvediev V, Abdallah I, Bozhenko A, Tatarchuk M, Ishchenko Y, Pichkur L, Savosko S, Tsymbaliuk V, Kopach O, Voitenko N. Phenotypes of Motor Deficit and Pain after Experimental Spinal Cord Injury. Bioengineering (Basel). 2022 Jun 20;9(6):262. doi: 10.3390/bioengineering9060262. PMID: 35735505.
- Krotov V, Agashkov K, Krasniakova M, Safronov BV, Belan P, Voitenko N. Segmental and descending control of primary afferent input to the spinal lamina X. Pain. 2022 Oct 1;163(10):2014-2020. doi: 10.1097/j.pain.0000000000002597. Epub 2022 Jan 31. PMID: 35297816.
- Duzhyy DE, Voitenko NV, Belan PV. Peripheral Inflammation Results in Increased Excitability of Capsaicin-Insensitive Nociceptive DRG Neurons Mediated by Upregulation of ASICs and Voltage-Gated Ion Channels. Front Cell Neurosci. 2021 Oct 18;15:723295. doi: 10.3389/fncel.2021.723295. PMID: 34733139.
- Transcutaneous endoscopic gastrostomy: indications, technique, complications and results / T. V. Bukharin, V. A. Yakovenko, Yu. V. Flomin, M. A. Mendel. Klinicheskaia khirurgiia. 2018. Vol. 85 (8). P. 21-25. DOI: 10.26779/2522-1396.2018.08.21.
- Kryzhevskii V. V., Mendel N. A., Pavlovich Yu. V. Technical features of laparoscopic minicholecystectomy. Klinicheskaia khirurgiia. 2018. Vol. 85 (11.2). P. 32-35.
- Kryzhevskii V. V., Mendel N. A., Pavlovich Yu. V. Techniques for achieving safe laparoscopic cholecystectomy (Review). Klinicheskaia khirurgiia. 2019. №11.2. P.37-42.
- Prophylaxis of infection in region of the surgical intervention performance in laparoscopic cholecystectomy / V. V. Kryzhevskii, N. A. Mendel, A. P. Brodskaya, Yu. V. Pavlovich. Klinicheskaia khirurgiia. 2020. Vol. 87 (3-4). P. 22-25. DOI: 10.26779/2522-1396.2020.3-4.22.
- Golovko A., Kuryk O., Tkachenko R., Mendel N. Thyroid metastasis of vaginal leiomyosarcoma: a case report and review of the literature. Experimental oncology. 2020. Vol. 42, № 3. P.242–244. DOI: 10.32471/exp-oncology.2312-8852.vol-42-no-3.15029.
- Shkurupii D. A. Expression of the Toll-like receptors 2 gene as a genetic determinant of the formation of newborns multiple organ failure syndrome. The Turkish journal of pediatrics. 2019. № 61(4). Р. 500-504. doi: 10.24953/turkjped.2019.04.005.
- Dmytro Kholod, Dmytro Shkurupii. Gastrointestinal insufficiency syndrome in intensive care of newborn: literature review. Wiadomości lekarskie. 2019. № 72 (11 cz 1). Р. 2182-2186.
- Dmytro Shkurupii. Prevention of ventilator-associated pneumonia in newborns. Wiadomości lekarskie. 2018. № 71 (4). Р. 821-823.
- Dmytro Shkurupii. Early enteral nutrition as a part of intencive care of abdominal surgical pathology. Wiadomości lekarskie. 2017. № 70 (4). Р. 758-761.
- Dmytro Shkurupii. Nutritive support for newborns in critical conditions: semi-elemental formilas as a means of enteral nutrition. Wiadomości lekarskie. 2018. № 71 (2 pt 2). Р. 266-270.
- Kholod D., Shkurupii D., Sonnik Е. Immune changes in newborn infants with gastrointestinal failure requiring intensive care. Georgian medical news. 2016. № 256-257. Р. 62-66.
- Shkurupiĭ D. A., Kholod D. A. Sodium succinate as method of intensive care optimization of newborns’ multiorgan failure syndrom. Likars’ka sprava. 2014. № 7-8. Р. 76-80.
- Petro Teriv, Dmytro Shkurupii, Yuliia Hryshko. Condition and consequences of zinc metabolic disorder in patients with neurosurgical pathology requiring intensive care. Wiadomości lekarskie. 2016. № 69 (6). Р. 726-729.
- Shkurupiĭ D. A., Kutsenko N. L., Mamontova T. V. Immune elements of pathogenesis of multiorgan failure syndrome in newborns. Likars’ka sprava. 2013. № 3. Р. 93-96.
- Kholod D. A., Shkurupiĭ D. A. Ophthalmological disorders after spinal anesthesia. Anaesthesia, Pain & Intensive Care. 2021. № 25 (6). Р. 703-706. DOI: 10.35975/apic.v25i6.1696.
- Shkurupii D. A., Kholod D. A. Formation of a true knot in subclavian venous catheter in a patient in the intensive care unit. Anaesthesia, Pain & Intensive Care. 2020. Vol. 24, № 6. P. 664-666.
- Shkurupii D. A., Harkavenko M. О., Kholod D. A. Ophthalmological complications of general anesthesia. Novosti Khirurgii. 2018. Vol. 26 (1). P. 66-71.
- Shkurupiĭ D. A. Injuries caused by a military explosive device to children. Anaesthesia, Pain & Intensive Care. 2018. Vol. 22, № 1. Р. 101-104.
- Management of patients with chronic retinal diseases in the current conditions of the COVID -19 pandemic in the world and Ukraine / P. Bezditko, I. Bezkorovayna, D. Zhaboedov, N. Lutsenko, A. Sergienko, N. Ulyanova, M. Karliychuk, O. Pavliv, V. Shevchik. Journal of Ophthalmology (Ukraine). 2021. № 2 (499). Р. 82-85.
- International Practice Patterns for the Management of Acute Postsurgical and Postintravitreal Injection Endophthalmitis: European Vitreo-Retinal Society Endophthalmitis Study Report 1 / M. K. Soliman, G. Gini, F. Kuhn, A. Sergienko, Z. Aktas, Z. Szijártó. Ophthalmology Retina. 2019. № 3(6). P. 461–467.
- OCT angiography for diagnosing and postoperative monitoring of serous maculopathy associated with optic disc pit / N. S. Lutsenko, O. A. Rudycheva, O. A. Isakova, A. N. Sergienko. Ophthalmological Journal. 2019. № 1. С. 56-60.
- Comparative cyto-histological study of needle tip aspirates and entry sites after intravitreal injection using different needle types / L. Lytvynchuk, A. Sergienko, I. Savytska, S. Binder, G. Petrovski et al. PLoS ONE. 2017. № 6. 12(7). e0174467.
- Effect of cryopreserved placental mesenchymal stromal cells on pro-inflammatory state in experimental type 2 diabetes mellitus / Y. A. Demin, M. Y. Demina, V. A. Shabliy, A. N. Sergienko. Problems of Cryobiology and Cryomedicine. 2015. № 25 (4). Р. 371-378.
- Lytvynchuk L. M., Sergiienko A., Richard G. Modified curved aspiration cannulas and end-gripping forceps for 25-gauge vitrectomy on highly myopic eyes. Retina. 2015. № 35 (12). Р. 2669-2671.
- Antiproliferative, Apoptotic, and Autophagic Activity of Ranibizumab, Bevacizumab, Pegaptanib, and Aflibercept on Fibroblasts: Implication for Choroidal Neovascularization / L. Lytvynchuk, A. Sergienko, G. Lavrenchuk, G. Petrovski. Journal of Ophthalmology. 2015. Vol. 2015. Article ID 934963. doi: 10.1155/2015/934963.

